A place to discuss hardware/software and diagnostic procedures
Testing for ethanol fuel
- Tyler
-
 Topic Author
Topic Author
- Offline
- Moderator
-

- Full time HACK since 2012
Less
More
- Posts: 6080
- Thank you received: 1537
7 years 5 months ago #22671
by Tyler
Testing for ethanol fuel was created by Tyler
Doing this thread to enlighten anyone else that hasn't run into this problem before. Probably old hat to most of the pros around here, but the DYI'ers might get something out of this. 
Depending on where you live, ethanol fuel may be available at the pumps, and your customer may accidentally dump some into their tank. It happens, and fuel quality may not be high on your list of things to check. It's not for me, anyway. :lol: On a diesel? Sure, I'll take a sample. But gas is usually good.
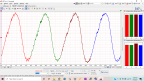
On a non-Flex vehicle, you'll almost certainly end up with lean codes when the tank is filled with E85. The vehicle in this example ('08 Saturn Aura) had scan data that looked like this:
www.scanshare.io/share/Vs8hyHJIAkSGfZeqZY7-Mg
My takeaway from the scan data was the fuel trims being elevated a (relatively) equal amount across all load ranges. Idle, cruise, acceleration. The trims were skewed positive around 30% the whole time. This eliminates a vacuum leak, and mostly eliminates a MAF. FWIW, the MAF was reporting around 5.6 g/s at hot idle with no loads. According to the g/s = engine displacement in liters rule, thats overreporting, not under, and thus won't account for a lean condition at idle.
If you're working at a GM dealer, you might have access to one of these:
But you probably don't, so you'll need another method to test for ethanol content. The basic idea is this: Take a fuel sample, put a known quantity in a clear container, and add a known quantity of water to the fuel. Skake it up and watch where the separation happens. The water will absorb the ethanol in the fuel, and thus sink to the bottom along with the water. For this example, I added nine parts gas and one part water to a gallon jug. This is a known good sample from my car:
The separation line is way down near the bottom, indicating a 15%-ish ethanol content. Right where it should be, if the pump is telling the truth. Now, the E85:
The separation line is much closer to the top. I didn't get scientific trying to figure out the exact percentage - this is obviously way too much ethanol for a non-Flex car.
Measuring out exactly nine parts fuel and one part water wasn't too critical in this example. But, I can see how it would be if you were dealing with half a tank of E10 that got topped off with E85. I'd suggest being as exact as you reasonably can be.
Depending on where you live, ethanol fuel may be available at the pumps, and your customer may accidentally dump some into their tank. It happens, and fuel quality may not be high on your list of things to check. It's not for me, anyway. :lol: On a diesel? Sure, I'll take a sample. But gas is usually good.
On a non-Flex vehicle, you'll almost certainly end up with lean codes when the tank is filled with E85. The vehicle in this example ('08 Saturn Aura) had scan data that looked like this:
www.scanshare.io/share/Vs8hyHJIAkSGfZeqZY7-Mg
My takeaway from the scan data was the fuel trims being elevated a (relatively) equal amount across all load ranges. Idle, cruise, acceleration. The trims were skewed positive around 30% the whole time. This eliminates a vacuum leak, and mostly eliminates a MAF. FWIW, the MAF was reporting around 5.6 g/s at hot idle with no loads. According to the g/s = engine displacement in liters rule, thats overreporting, not under, and thus won't account for a lean condition at idle.
If you're working at a GM dealer, you might have access to one of these:
But you probably don't, so you'll need another method to test for ethanol content. The basic idea is this: Take a fuel sample, put a known quantity in a clear container, and add a known quantity of water to the fuel. Skake it up and watch where the separation happens. The water will absorb the ethanol in the fuel, and thus sink to the bottom along with the water. For this example, I added nine parts gas and one part water to a gallon jug. This is a known good sample from my car:
The separation line is way down near the bottom, indicating a 15%-ish ethanol content. Right where it should be, if the pump is telling the truth. Now, the E85:
The separation line is much closer to the top. I didn't get scientific trying to figure out the exact percentage - this is obviously way too much ethanol for a non-Flex car.
Measuring out exactly nine parts fuel and one part water wasn't too critical in this example. But, I can see how it would be if you were dealing with half a tank of E10 that got topped off with E85. I'd suggest being as exact as you reasonably can be.
The following user(s) said Thank You: Noah, Andy.MacFadyen, Chad
Please Log in or Create an account to join the conversation.
- Chad
-

- Offline
- Moderator
-

- I am not a parts changer.
Less
More
- Posts: 2141
- Thank you received: 721
7 years 5 months ago #22673
by Chad
"Knowledge is a weapon. Arm yourself, well, before going to do battle."
"Understanding a question is half an answer."
I have learned more by being wrong, than I have by being right.
Replied by Chad on topic Testing for ethanol fuel
I take a, very, similar approach. I don't worry about knowing how much water I start with. I fill a clear container about 25% full with water. (The amount of water is only important in that there is enough to absorb all of the alcohol.) Mark the water level with a Sharpie. Then, Mark out 10 equal segments above the water level. Slowly fill the container the rest of the way to the top of the last mark.
Then Shake.
This gas is 10% ethanol.
Then Shake.
This gas is 10% ethanol.
"Knowledge is a weapon. Arm yourself, well, before going to do battle."
"Understanding a question is half an answer."
I have learned more by being wrong, than I have by being right.
The following user(s) said Thank You: Noah, Andy.MacFadyen
Please Log in or Create an account to join the conversation.
- Andy.MacFadyen
-

- Offline
- Moderator
-

Less
More
- Posts: 3353
- Thank you received: 1037
7 years 5 months ago - 7 years 5 months ago #22676
by Andy.MacFadyen
" We're trying to plug a hole in the universe, what are you doing ?. "
(Walter Bishop Fringe TV show)
Replied by Andy.MacFadyen on topic Testing for ethanol fuel
Not a problem we see yet here in the UK because the various automotive interest groups have slowed the forced adoption of higher alcohol content fuels but a subject I have been following closely.
Here is Paul's Youtube video on the issue
Here is Paul's Youtube video on the issue
" We're trying to plug a hole in the universe, what are you doing ?. "
(Walter Bishop Fringe TV show)
Last edit: 7 years 5 months ago by Andy.MacFadyen.
Please Log in or Create an account to join the conversation.
Time to create page: 0.299 seconds





